BionanomatrixTM Applications in Development
| Application | Formulation | Discovery | Preclinical | IND-Enabl. | Clinical | Market | Partners |
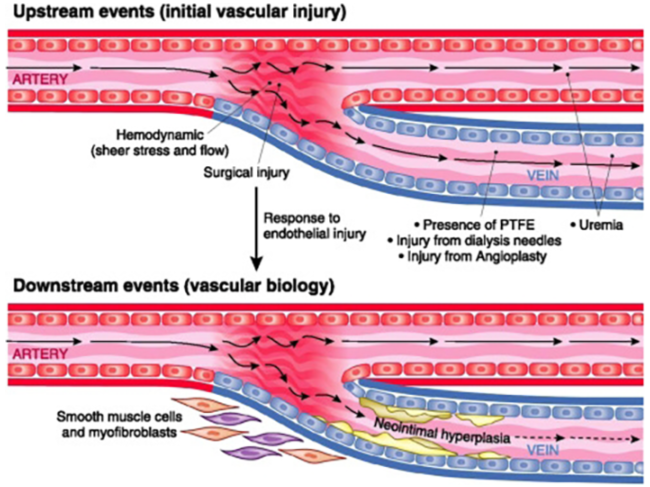
| Arteriovenous fistula | Gel, NO |

|

|

|
$1.3B by 2032 | ||
| Cardiovascular stents | Coating, NO |

|

|
$15.6B by 2030 | |||
| Flow diverters | Coating, NO |

|

|
$1.3B by 2032 | |||
| Angioplasty balloons | Coating, sirolimus+NO |

|

|
$3.3B by 2028 | |||
| Direct pulp capping | Coating, NO |

|

|
$1.9B by 2031 |

|
||
| Knee Replacement | Coating, NO |

|

|
$14.5B by 2032 |
Arteriovenous Fistula Market Outlook 2022-2032, Future Market Insights
Cardiovascular Stent Market Research Future, December 2022
Flow Diverter Market Outlook 2022-2032, Future Market Insights
Angioplasty Balloon Market - Growth, Trends, COVID-19 Impact, and forecasts (2023-2028)
Dental Cavity Filling Material Market - Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2022-2031
Knee Replacement Market - Global Market Insights, Report ID: GM12804, 2022